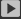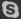The ideal solvent suitable for lithium ion batteries should have a high oxidation potential and a low reduction potential. Table 2-6 is an XU summary of a lithium-ion battery commonly solvent carbonate, butin, ether, ether, oxidation or decomposition potential. As can be seen from the table. Carbonate or other lipid substances have high anode stability, and the cathode electrochemical stability of the ether is relatively high. For example, the oxidative potential of the carbonate is greater than 4.2V, and the oxidation potential of the ether is generally less than 4.2V and is easily A polymerization occurs, so at lower voltages, the ether may decompose, causing a decrease in battery energy, and Liu's electricity is unfavorable. In addition, some solvents (such as diethyl ether, cyclobutyl ether, oadala) containing a strong polar functional group have very high stability, such as an electrochemical window person of cyclobutolane, about 6.1 V.
The ideal solvent suitable for lithium ion batteries should have a high oxidation potential and a low reduction potential. Table 2-6 is an XU summary of a lithium-ion battery commonly solvent carbonate, butin, ether, ether, oxidation or decomposition potential. As can be seen from the table. Carbonate or other lipid substances have high anode stability, and the cathode electrochemical stability of the ether is relatively high. For example, the oxidative potential of the carbonate is greater than 4.2V, and the oxidation potential of the ether is generally less than 4.2V and is easily A polymerization occurs, so at lower voltages, the ether may decompose, causing a decrease in battery energy, and Liu's electricity is unfavorable. In addition, some solvents (such as diethyl ether, cyclobutyl ether, oadala) containing a strong polar functional group have very high stability, such as an electrochemical window person of cyclobutolane, about 6.1 V.All reduction potentials for solvent components for lithium ion battery electrolytes are higher than those of metal lithium electrode potential. They are reduced in the negative surface during battery charge and discharge. For example, ZHANG, etc., using cyclic voltammetry as a support electrolyte in 0.Lmol / L LiClO4 / THF6, and the reduction process of EC. PC, DEC, DMC, and VC in gold and other inert electrode surfaces were studied in detail. The study found that the reduction voltages of EC, VC and PC were 1.6V, 1.4V, 1.6V, respectively. The different reduction potentials actually determine the composition of the surface passivation film of the electrode. This has an important influence on the compatibility of the electrolyte and the electrode.
In a lithium ion battery, in order to obtain a higher single cell voltage, the positive electrode material often tends to select a high potential lithium compound. Such as LiCoO2, LiniO2, UMN 204, etc. These electrode materials have different effects on the oxidative properties of the electrolyte. Figure 2-13 shows the oxidative potential of linear carbonate DMC and EMC at the glass carbon electrode 1: 6.0 V.80]. However, in spinel I.IMN2O. As the 1 binary electrodes, the two can be oxidized in 4.5V. The electrode material of this oxidation potential cannot simply be considered to be a foot LiMN204 electrode table, and more important causes may be the catalysis of electrode surface active substances to the electrolysis process.
When IMHOF, when the LINIO2 is positive, it can be decomposed when the voltage is 4.2V (vs.li + / li), and there is a decomposition of the Solvent of the case. There is carbon dioxide gas. However, if LiCoO2 or LiMN2O is used as the working electrode, it may have the same phenomenon.
The stability of the partial electrolyte system in different in-element lithium compounds in different inline compounds. It is not difficult to see from the table, the stability of the thiraminated electrolyte is high. The stability of the lipid electrolyte is generally higher than the ether. Figure 2-14 is a voltammer of the spinel LiMn2O4 electrode material in EC Ten DEE and EC Ten DMC electrolyte at 55 ° C. As can be seen from the figure. 3. 9V and 4. 1V two oxidation peaks correspond to the escape of lithium-离 plants, respectively. When the voltage exceeds 4. 4V.1. Otnol / llicio., / EC + DEE 解 解 解 l l l (Electrochemical Stability of '+ II Electrode Materials 1. Omol / L 1.IPFR / EC '+ I) M. Even if the current density is still small even at 5V, it indicates that its antioxidant properties are very good, so the use of linear lipids is more advantageous to improve battery safety. Recommend: LiFePO4 Battery Manufacturer Energy storage battery Manufacturer Integrated machine energy storage battery series Manufacturer Lead lithium battery Manufacturer Outdoor Backup Battery Manufacturer Portable outdoor power supply Manufacturer Power battery Manufacturer Powerwall LiFePO4 Battery Manufacturer Battery rack Manufacturers Telecom LiFePO4 Battery Manufacturer Wall mounted battery storage Manufacturer China Lifepo4 Battery