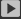The lithium ion battery electrolyte is prone to decomposition reactions during charge and discharge, and is accompanied by heat, and the long-term use of lithium ion batteries has brought safe hidden dangers, and it also brought difficult to the commercialization of large volume lithium ion batteries.
The lithium ion battery electrolyte is prone to decomposition reactions during charge and discharge, and is accompanied by heat, and the long-term use of lithium ion batteries has brought safe hidden dangers, and it also brought difficult to the commercialization of large volume lithium ion batteries.For example, as a lithium ion battery of the automotive energy requires a large charge and discharge current, high power, which will generate a high temperature in part. In general, the organic liquid electrolyte is difficult to maintain stability under such conditions. Therefore, the thermal stability of the organic electrolyte is an important factor in the development and study of lithium-ion batteries.
The study of the thermal stability of organic electrolytes mainly includes two aspects: the thermal stability of the organic electrolyte itself and the thermal stability when the electrolyte is interacting with the electrode material. The former is determined by its own nature, and the latter is related to the electrode properties, relatively more complicated. Figure 2-23 is a thermogravimetric analysis of six lithium ion batteries commonly used lithium salts C933. It is not difficult to see from the figure that LiPF6 begins to decompose at low temperatures, Libf4, and the radioceneability of LiCl04 is preferably, and the decomposition temperature of LIN (S02CF3) 2 and LIN (SOZC2FS) 2 is greater than 300 ~ C, with fluorine The increase in the length of the alkyl chain length is slightly lowered.
High purity LiPF6 under sealing conditions at 194 to c, there is a differential hot peak, and the temperature is continued to decompose from 250 to C. The first difference heat peak is caused by the transformation of the LIPF6. When LiPF6 heated under the conditions of flowing high purity argon and LMG / kg, 10 h at 85 ¡ã C at 85 ¡ã C was lost 20% a 30%.
If 20 to c begin to decompose under low purity argon conditions, the product is primarily PFS and LIF. Obviously, the LIPF6 is the worst, and the storage conditions are most demanding compared to other lithium salts. In addition, LiPFS also has very strong humidity, and decomposes the formation of HF and other substances. These decomposition products (especially PFS) are easily reacted with the carbonate solvent, destroy the electrolyte, and is the main reason for the unstable electrolyte composition of LiPF6 and mixed carbonate Recommend: LiFePO4 Battery Manufacturer Energy storage battery Manufacturer Integrated machine energy storage battery series Manufacturer Lead lithium battery Manufacturer Outdoor Backup Battery Manufacturer Portable outdoor power supply Manufacturer Power battery Manufacturer Powerwall LiFePO4 Battery Manufacturer Battery rack Manufacturers Telecom LiFePO4 Battery Manufacturer Wall mounted battery storage Manufacturer China Lifepo4 Battery